RESEARCH
 Load apparatus contains 12 sample wells |
|---|
 Unconfined loading |
 Confined loading |
Effects of mechanical loads on
scaffold-cartilage integration
Cartilage has limited healing potential, due to slow metabolism and the lack of blood supply. One of treatment options is utilizing scaffold as a cartilage substitue to replace focal defects.
While scaffolds have been developed to restore structure and function of damaged tissue, their main limitation has been an inability to integrate with host cartilage in a robust maner. Beneficial effects of mechanical loads, in physiological levels, have been studied in animals and tissue engineered constructs and shown that mechanical loads improved metrix production, thickness and mechanical properties of cartilage.
Currently, we do not have a constructive information of mechanical loads on integrative process, though articular cartilage experiences mechanical environment in moving joints. In this project, we explore the effect of controlled loads across cartilage explants and study scaffold-cartilage integrative abilities.
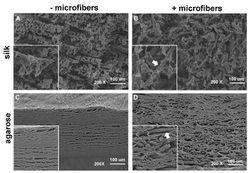 SEMs of fiber-reinforced hydrogelsSilk hydrogels adhered to silk microfibers (top right), but agarose hydrogels clearly separated from the fibers (bottom right) |
|---|
 Silk hydrogelmicrofiber reinforced-engineered cartilage: GAG staining located around silk fibers |
 Electrospun nanofibersThe nanofibers were made from Poly(Vinyl) Alcohol, PVA |
Fiber-reinforced Hydrogel for Cartilage Tissue Engineering
Cartilage is a connective tissue composed of chondrocytes dispersed in a dense extracellular matrix (ECM). The hydrophilic environment of ECM, due to negatively charged glycosaminoglycans that attract water, enables cartilage tissues to experience a swelling pressure, which is countered by tensile strength generated from the interspersed collagen network.
We are interested in improvement of hydrogel mechanics by combining a dual polymeric system in which one material serves as the gel network while the other is interspersed within the gel as overlapping fibers.
 Embryoid Bodies (EBs)Embryoeid bodies were cultured in low oxygen tension at 5%. bar = 200 um |
|---|
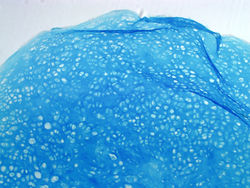 Chondrogenic pelletsGAG staining of chondrogenic pellets derived from hESCs |
 Stem cells to chondrogenic pelletsHuman embryonic stem cells (hESCs) and embryos bodies (EBs) during chondrogenic differentiation |
Hypoxia and Chondrogenic Differentiation
Native adult articular cartilage is a physiologically hypoxic tissue, with oxygen tension ranging from <10% at the cartilage surface to <1% in the deepest layers. Beside the spatial gradients, there is also a temporal change of oxygen tension that occurs during cartilage development. Still, a vast majority of the cartilage tissue engineering protocols involves cultivation of chondrocytes or their progenitors under ambient oxygen concentration (21% O2), which is significantly higher than the oxygen levels in native joints.
To mimick oxygen levels during limb development, chondrocytes or human embryonic stem cells were exposed to low oxygen tension to promote chondrogenic comitment. We hypothesized that the properties of tissue engineered cartilage could be improved if oxygen in culture medium is initially maintained at physiologically low level (5%, to enhance chondrogenic differentiation) and then increased to ambient level (21%, to enhance collagen synthesis and functional assembly).
Native adult articular cartilage is a physiologically hypoxic tissue, with oxygen tension ranging from <10% at the cartilage surface to <1% in the deepest layers. Beside the spatial gradients, there is also a temporal change of oxygen tension that occurs during cartilage development. Still, a vast majority of the cartilage tissue engineering protocols involves cultivation of chondrocytes or their progenitors under ambient oxygen concentration (21% O2), which is significantly higher than the oxygen levels in native joints.
To mimick oxygen levels during limb development, chondrocytes or human embryonic stem cells were exposed to low oxygen tension to promote chondrogenic comitment. We hypothesized that the properties of tissue engineered cartilage could be improved if oxygen in culture medium is initially maintained at physiologically low level (5%, to enhance chondrogenic differentiation) and then increased to ambient level (21%, to enhance collagen synthesis and functional assembly).

scaffold for cartilage
tissue engineering
Dense extracellular matrix of articular cartilage is a natural barrier that prevents scaffold-cartilage integration. We propose the alternative approach to enhance a stable interface between scaffold and cartilage tissue using mechanical interlock rather than chemical-based adhesive glue. Polymers will be used to fabricate a biphasic scaffold with i) porous core and ii) microneedle periphery. The needles which, when attached to a porous core, are intended to mechanically interlock the scaffold with the cartilage tissue into which it is implanted. To explore this concept, microneedles will be fabricated and attached on the porous core. The biphasic scaffold will be tested for cell attachment and viability. The challenge of this project is the control of microneedle stiffness where it has to puncture the cartilage tissue and be surrounded by collagen network, similar to hook-loop configuration in Velcro. We will study integrative capability of the biphasic scaffold in cartilage explant using a defect-repair model. This novel scaffold is aimed at improving treatment for debilitating cartilage injuries.